Please log in to read this in our online viewer!
Please log in to read this in our online viewer!
No comments yet. You can be the first!
Content extract
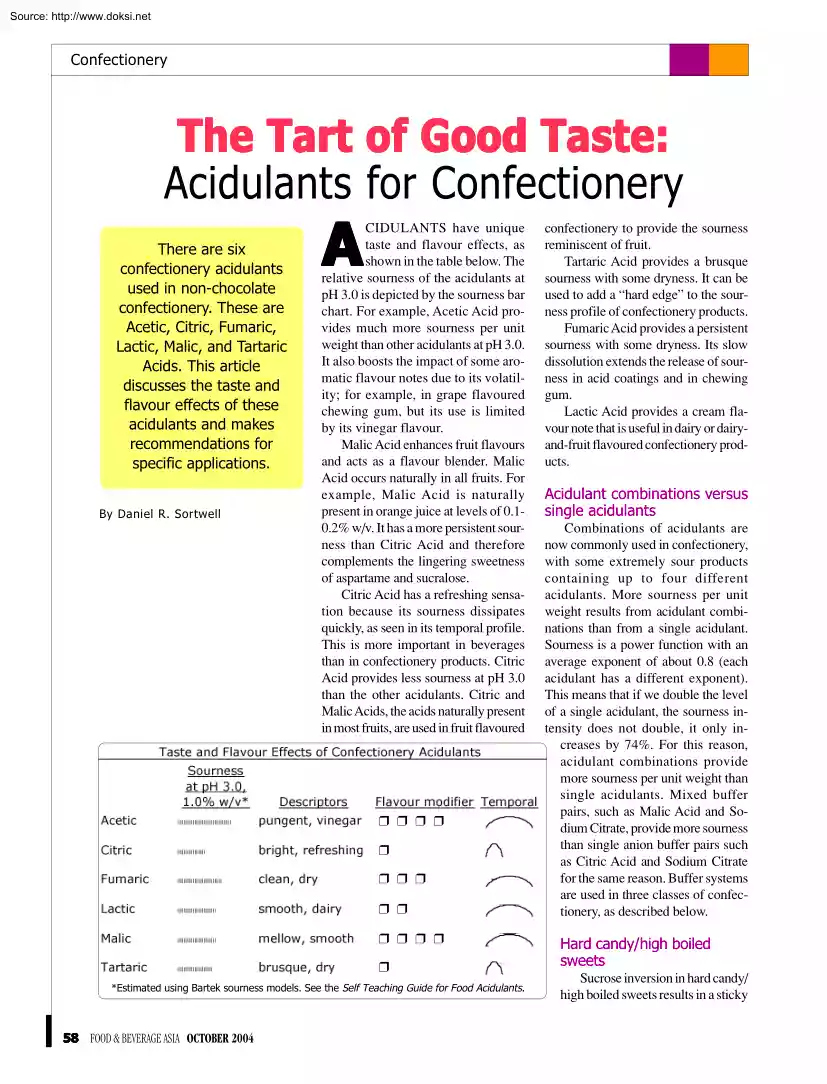
Source: http://www.doksinet Confectionery The Tart of Good Taste: Acidulants for Confectionery There are six confectionery acidulants used in non-chocolate confectionery. These are Acetic, Citric, Fumaric, Lactic, Malic, and Tartaric Acids. This article discusses the taste and flavour effects of these acidulants and makes recommendations for specific applications. By Daniel R. Sortwell A CIDULANTS have unique taste and flavour effects, as shown in the table below. The relative sourness of the acidulants at pH 3.0 is depicted by the sourness bar chart. For example, Acetic Acid provides much more sourness per unit weight than other acidulants at pH 3.0 It also boosts the impact of some aromatic flavour notes due to its volatility; for example, in grape flavoured chewing gum, but its use is limited by its vinegar flavour. Malic Acid enhances fruit flavours and acts as a flavour blender. Malic Acid occurs naturally in all fruits. For example, Malic Acid is naturally present in orange
juice at levels of 0.102% w/v It has a more persistent sourness than Citric Acid and therefore complements the lingering sweetness of aspartame and sucralose. Citric Acid has a refreshing sensation because its sourness dissipates quickly, as seen in its temporal profile. This is more important in beverages than in confectionery products. Citric Acid provides less sourness at pH 3.0 than the other acidulants. Citric and Malic Acids, the acids naturally present in most fruits, are used in fruit flavoured ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ *Estimated using Bartek sourness models. See the Self Teaching Guide for Food Acidulants 58 FOOD & BEVERAGE ASIA OCTOBER 2004 confectionery to provide the sourness reminiscent of fruit. Tartaric Acid provides a brusque sourness with some dryness. It can be used to add a “hard edge” to the sourness profile of confectionery products. Fumaric Acid provides a persistent sourness with some dryness. Its slow dissolution
extends the release of sourness in acid coatings and in chewing gum. Lactic Acid provides a cream flavour note that is useful in dairy or dairyand-fruit flavoured confectionery products. Acidulant combinations versus single acidulants Combinations of acidulants are now commonly used in confectionery, with some extremely sour products containing up to four different acidulants. More sourness per unit weight results from acidulant combinations than from a single acidulant. Sourness is a power function with an average exponent of about 0.8 (each acidulant has a different exponent). This means that if we double the level of a single acidulant, the sourness intensity does not double, it only increases by 74%. For this reason, acidulant combinations provide more sourness per unit weight than single acidulants. Mixed buffer pairs, such as Malic Acid and Sodium Citrate, provide more sourness than single anion buffer pairs such as Citric Acid and Sodium Citrate for the same reason. Buffer
systems are used in three classes of confectionery, as described below. Hard candy/high boiled sweets Sucrose inversion in hard candy/ high boiled sweets results in a sticky Source: http://www.doksinet Confectionery provide sourness (an essential elsurface that is unacceptable. To reement of fruit taste) and to stimutard sucrose inversion: “Sourness is a power function with an late saliva flow. 1. Cool down the acidified average exponent of about 0.8 This Acidulant combinations are molten hard candy as rapidly as means that if we double the level of a used in so that sourness will be possible. single acidulant, the sourness intensity perceived over a longer time pe2. Minimise the initial moisdoes not double, it only increases by riod, since acidulants differ in ture level of the candy. their rate of sourness release in 3. Use acid/buffer salt com74% For this reason, acidulant chewing gum systems. The rate binations to raise the pH. Combicombinations provide more sourness nations
of Malic Acid and Sodium per unit weight than single acidulants.” of release is influenced by the hydrophobicity of the acidulant, Citrate or Malic Acid and Sodium usually measured by the partition Lactate provide more sourness and buffer capacity than other buffer pairs at pH 3.0 These coefficient Since gum base is hydrophobic, acidulants that are more buffer pairs also take advantage of Malic Acid’s fruit flahydrophobic associate more strongly with the gum base and vour-enhancing property. Liquid acid/buffer salt solutions are metered into con- release more slowly. The hydrophobicity of the gum base tinuous process systems. A Malic Acid and Sodium Lactate itself or of other ingredients in the chewing gum may also solution for continuous metering consists of: 59% water, 27% be adjusted to strengthen the association with a particular acidulant in order to delay its release. Malic Acid, and 14% Sodium Lactate. The most hydrophobic of the food acidulants is Fumaric For hard candy
made with isomalt, acidulants with persistent sourness, such as Malic and Lactic Acids, are used to Acid. Powdered Fumaric Acid is used in some chewing gums to prolong sourness. In the manufacture of compressed chewmatch the slightly delayed sweetness of isomalt ing gum tablets, process yields and efficiencies are improved by using more hydrophobic acidulants, since these are more Chewing gum Acidulants are used in fruit flavoured chewing gum to effective tablet lubricants. FOOD & BEVERAGE ASIA OCTOBER 2004 59 Source: http://www.doksinet Confectionery “To retard sucrose inversion in candies, the initial moisture level of the product should be minimised. Acid/buffer salt combinations are used to raise the pH. Acids coated with vegetable fat or buffer salts also prevent the interaction between the acids and sucrose.” Confectionery jellies Acid hydrolysis of hydrocolloids results in a loss of gel strength and in gel texture variation. To minimise acid hydrolysis of
hydrocolloids in confectionery jellies: 1. Add the acid last and cool down the acidified product as rapidly as possible. 2. Use acid/buffer salt combinations to raise the pH The acid/buffer salt combination for maximum sourness and buffer capacity depends on the target pH. At a pH below 35, the combination of Citric Acid and Sodium Citrate provides less sourness and buffer capacity than combinations of Malic Acid with Sodium Citrate, Malic Acid with Sodium Lactate, or Lactic Acid with Sodium Lactate. Limiting pH variation to a narrow range controls gel texture. This is done using buffer systems. Gelatine bloom strength is reduced when both glucose syrups and buffer salts are present. Compressed tablets Both Malic and Fumaric Acids provide significantly more sourness per unit weight than Citric Acid at pH 3.0 It is possible to reduce the acid level in the tablet by using these acids. Malic Acid enhances fruit flavours in compressed tablets and its persistent sourness complements the
lingering sweetness of aspartame and 60 FOOD & BEVERAGE ASIA OCTOBER 2004 sucralose. Malic and Fumaric Acids improve tablet lubrication compared to Citric or Tartaric Acid because they are more hydrophobic. This results in a tablet that is less brittle and that has a smoother surface. This is especially important for engraved tablets. Process efficiencies are also improved because there is less tablet breakage and less tablet adhesion to the punch face and diewall. Acid sanded confectionery The challenge in acid sanded confectionery is to prevent sucrose inversion from starting, because once it starts, it is difficult, if not impossible, to stop. Sucrose inversion in acid sanded confections results in a sticky surface that is unacceptable. To retard sucrose inversion: 1. Use acid/buffer salt combinations to raise the pH Combinations of Malic Acid and Sodium Citrate or Malic Acid and Sodium Lactate provide more sourness and buffer capacity than other buffer pairs at pH 3.0 These
buffer pairs also take advantage of Malic Acid’s fruit flavour-enhancing property. 2. Use coated acids to prevent the interaction between the acids and su- crose. Vegetable fat coatings are normally used for this application One manufacturer also coats acid particles with buffer salts. 3. Use Fumaric Acid, which is nonhygroscopic, as part of the coating. 4. Separate the acid and sucrose by using a coating that contains only acid and no sucrose. 5. Use an anti-caking agent as part of the coating to control excess moisture. 6. Thoroughly dry the sanded confection 7. Use conditioned air in the packaging area Trends in confectionery acidulants Many low carbohydrate confectionery products have been launched in North America. These products use sweeteners such as aspartame, sucralose and isomalt. To achieve the optimum sweet/sour balance in these products, the acidulant or acidulant combination is adjusted to mirror the temporal profile of the sweeteners used. Since these sweeteners are
more persistent than sucrose or fructose, it makes sense to use acidulants with lingering sourness such as Lactic, Fumaric, and Malic Acids in these products. Enquiry No: 043 Daniel R. Sortwell is Senior Food Scientist, Bartek Ingredients Inc Additional information about acidulants and their application in confectionery is found in the Self Teaching Guide for Food Acidulants, which is at Bartek’s web site: www.bartekca
juice at levels of 0.102% w/v It has a more persistent sourness than Citric Acid and therefore complements the lingering sweetness of aspartame and sucralose. Citric Acid has a refreshing sensation because its sourness dissipates quickly, as seen in its temporal profile. This is more important in beverages than in confectionery products. Citric Acid provides less sourness at pH 3.0 than the other acidulants. Citric and Malic Acids, the acids naturally present in most fruits, are used in fruit flavoured ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ ❒ *Estimated using Bartek sourness models. See the Self Teaching Guide for Food Acidulants 58 FOOD & BEVERAGE ASIA OCTOBER 2004 confectionery to provide the sourness reminiscent of fruit. Tartaric Acid provides a brusque sourness with some dryness. It can be used to add a “hard edge” to the sourness profile of confectionery products. Fumaric Acid provides a persistent sourness with some dryness. Its slow dissolution
extends the release of sourness in acid coatings and in chewing gum. Lactic Acid provides a cream flavour note that is useful in dairy or dairyand-fruit flavoured confectionery products. Acidulant combinations versus single acidulants Combinations of acidulants are now commonly used in confectionery, with some extremely sour products containing up to four different acidulants. More sourness per unit weight results from acidulant combinations than from a single acidulant. Sourness is a power function with an average exponent of about 0.8 (each acidulant has a different exponent). This means that if we double the level of a single acidulant, the sourness intensity does not double, it only increases by 74%. For this reason, acidulant combinations provide more sourness per unit weight than single acidulants. Mixed buffer pairs, such as Malic Acid and Sodium Citrate, provide more sourness than single anion buffer pairs such as Citric Acid and Sodium Citrate for the same reason. Buffer
systems are used in three classes of confectionery, as described below. Hard candy/high boiled sweets Sucrose inversion in hard candy/ high boiled sweets results in a sticky Source: http://www.doksinet Confectionery provide sourness (an essential elsurface that is unacceptable. To reement of fruit taste) and to stimutard sucrose inversion: “Sourness is a power function with an late saliva flow. 1. Cool down the acidified average exponent of about 0.8 This Acidulant combinations are molten hard candy as rapidly as means that if we double the level of a used in so that sourness will be possible. single acidulant, the sourness intensity perceived over a longer time pe2. Minimise the initial moisdoes not double, it only increases by riod, since acidulants differ in ture level of the candy. their rate of sourness release in 3. Use acid/buffer salt com74% For this reason, acidulant chewing gum systems. The rate binations to raise the pH. Combicombinations provide more sourness nations
of Malic Acid and Sodium per unit weight than single acidulants.” of release is influenced by the hydrophobicity of the acidulant, Citrate or Malic Acid and Sodium usually measured by the partition Lactate provide more sourness and buffer capacity than other buffer pairs at pH 3.0 These coefficient Since gum base is hydrophobic, acidulants that are more buffer pairs also take advantage of Malic Acid’s fruit flahydrophobic associate more strongly with the gum base and vour-enhancing property. Liquid acid/buffer salt solutions are metered into con- release more slowly. The hydrophobicity of the gum base tinuous process systems. A Malic Acid and Sodium Lactate itself or of other ingredients in the chewing gum may also solution for continuous metering consists of: 59% water, 27% be adjusted to strengthen the association with a particular acidulant in order to delay its release. Malic Acid, and 14% Sodium Lactate. The most hydrophobic of the food acidulants is Fumaric For hard candy
made with isomalt, acidulants with persistent sourness, such as Malic and Lactic Acids, are used to Acid. Powdered Fumaric Acid is used in some chewing gums to prolong sourness. In the manufacture of compressed chewmatch the slightly delayed sweetness of isomalt ing gum tablets, process yields and efficiencies are improved by using more hydrophobic acidulants, since these are more Chewing gum Acidulants are used in fruit flavoured chewing gum to effective tablet lubricants. FOOD & BEVERAGE ASIA OCTOBER 2004 59 Source: http://www.doksinet Confectionery “To retard sucrose inversion in candies, the initial moisture level of the product should be minimised. Acid/buffer salt combinations are used to raise the pH. Acids coated with vegetable fat or buffer salts also prevent the interaction between the acids and sucrose.” Confectionery jellies Acid hydrolysis of hydrocolloids results in a loss of gel strength and in gel texture variation. To minimise acid hydrolysis of
hydrocolloids in confectionery jellies: 1. Add the acid last and cool down the acidified product as rapidly as possible. 2. Use acid/buffer salt combinations to raise the pH The acid/buffer salt combination for maximum sourness and buffer capacity depends on the target pH. At a pH below 35, the combination of Citric Acid and Sodium Citrate provides less sourness and buffer capacity than combinations of Malic Acid with Sodium Citrate, Malic Acid with Sodium Lactate, or Lactic Acid with Sodium Lactate. Limiting pH variation to a narrow range controls gel texture. This is done using buffer systems. Gelatine bloom strength is reduced when both glucose syrups and buffer salts are present. Compressed tablets Both Malic and Fumaric Acids provide significantly more sourness per unit weight than Citric Acid at pH 3.0 It is possible to reduce the acid level in the tablet by using these acids. Malic Acid enhances fruit flavours in compressed tablets and its persistent sourness complements the
lingering sweetness of aspartame and 60 FOOD & BEVERAGE ASIA OCTOBER 2004 sucralose. Malic and Fumaric Acids improve tablet lubrication compared to Citric or Tartaric Acid because they are more hydrophobic. This results in a tablet that is less brittle and that has a smoother surface. This is especially important for engraved tablets. Process efficiencies are also improved because there is less tablet breakage and less tablet adhesion to the punch face and diewall. Acid sanded confectionery The challenge in acid sanded confectionery is to prevent sucrose inversion from starting, because once it starts, it is difficult, if not impossible, to stop. Sucrose inversion in acid sanded confections results in a sticky surface that is unacceptable. To retard sucrose inversion: 1. Use acid/buffer salt combinations to raise the pH Combinations of Malic Acid and Sodium Citrate or Malic Acid and Sodium Lactate provide more sourness and buffer capacity than other buffer pairs at pH 3.0 These
buffer pairs also take advantage of Malic Acid’s fruit flavour-enhancing property. 2. Use coated acids to prevent the interaction between the acids and su- crose. Vegetable fat coatings are normally used for this application One manufacturer also coats acid particles with buffer salts. 3. Use Fumaric Acid, which is nonhygroscopic, as part of the coating. 4. Separate the acid and sucrose by using a coating that contains only acid and no sucrose. 5. Use an anti-caking agent as part of the coating to control excess moisture. 6. Thoroughly dry the sanded confection 7. Use conditioned air in the packaging area Trends in confectionery acidulants Many low carbohydrate confectionery products have been launched in North America. These products use sweeteners such as aspartame, sucralose and isomalt. To achieve the optimum sweet/sour balance in these products, the acidulant or acidulant combination is adjusted to mirror the temporal profile of the sweeteners used. Since these sweeteners are
more persistent than sucrose or fructose, it makes sense to use acidulants with lingering sourness such as Lactic, Fumaric, and Malic Acids in these products. Enquiry No: 043 Daniel R. Sortwell is Senior Food Scientist, Bartek Ingredients Inc Additional information about acidulants and their application in confectionery is found in the Self Teaching Guide for Food Acidulants, which is at Bartek’s web site: www.bartekca




 When reading, most of us just let a story wash over us, getting lost in the world of the book rather than paying attention to the individual elements of the plot or writing. However, in English class, our teachers ask us to look at the mechanics of the writing.
When reading, most of us just let a story wash over us, getting lost in the world of the book rather than paying attention to the individual elements of the plot or writing. However, in English class, our teachers ask us to look at the mechanics of the writing.