Please log in to read this in our online viewer!
Please log in to read this in our online viewer!
No comments yet. You can be the first!
What did others read after this?
Content extract
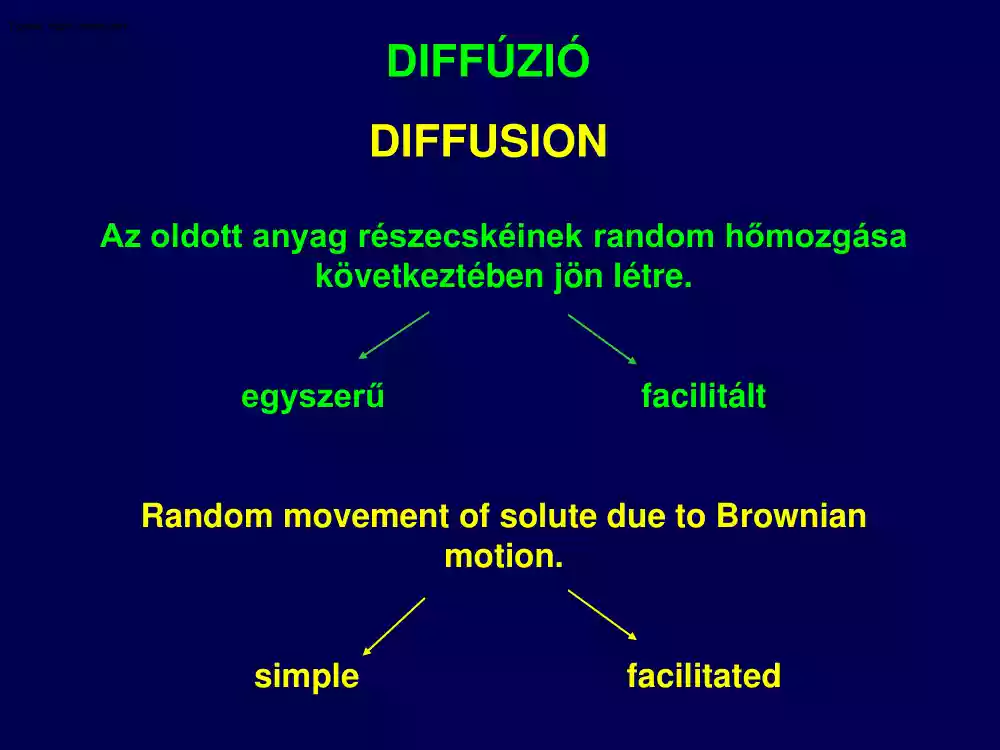
DIFFÚZIÓ DIFFUSION Az oldott anyag részecskéinek random hőmozgása következtében jön létre. egyszerű facilitált Random movement of solute due to Brownian motion. simple facilitated A tiszta foszfolipid kettősréteg áteresztőképessége szelektív A Pure Phospholipid Bilayer Acts as a Selectively Permeable Barrier Subtypes of Diffusion Transport of sodium and potassium ions through protein channels A diffúzióhoz szükséges idő a távolság függvényében Time Required for Diffusion to Occur over Various Diffusion Distances Az egyszerű és a facilitált diffúzió kinetikájának összehasonlítása Comparision of kinetics of simple and facilitated diffusion A Comparison of the Uptake Rate of Glucose by Facilitated Diffusion and Passive Diffusion Fick’s First Law of Diffusion J = -DmA (∆c/∆x) J: Net rate of diffusion in moles or grams per unit time Dm: Coefficient of molecular diffusion A: Area of the membrane ∆c:
Concentration difference across the membrane ∆x: Thickness of the membrane Például For example If the extracellular concentration of a substance doubles from 10 mM to 20 mM while the intracellular concentration remains at 5 mM, the rate of diffusion increases by how much? Twofold? Threefold? Fourfold? Például For example If the extracellular concentration of a substance doubles from 10 mM to 20 mM while the intracellular concentration remains at 5 mM, the rate of diffusion increases by how much? Threefold: (20-5):(10-5) or 15:5=3 !!! Ozmózis a sejtmembránon keresztül Osmosis at the Cell Membrane van’t Hoff’s Law π = RT(ϕic) π : Osmotic pressure R: Ideal gas constant T: Absolute temperature ϕ: Osmotic coefficient i: Number of ions formed by dissociation of a solute molecule c: Molar concentration of solute Osmolarity: The sum of the molarities of all dissolved particles in a solution - ϕic! Isotonic ~300 mosmol/l Hypotonic < 300 mosmol/l
Hypertonic > 300 mosmol/l Osmosis Nyugalmi membránpotenciál Resting membrane potential Dr. Zsembery Ákos Example 1 • Membrane permeable to K+, NOT to Cl• Transmembrane potential is zero and the net flux is zero. • Why? For each K+ ion that moves in one direction, another K+ ion moves in the opposite direction producing equal concentrations of K+ in I and II Example 2 • KCl added to I, creating a positive potential in II • 2 forces on K+ ions – Chemical gradient favoring net flux of K+ from I to II – Electrical potential difference favoring net flux of K+ from II to I Example 3 • Build-up of pos. charge in II repels movement of further K+ ions from I • Negative charges in I hold K+ back with electrostatic attraction • Electrochemical equilibrium is reached and no net flux of K+ occurs • Equilibrium potential for K+ is established across the membrane Nernst egyenlet Nernst equation Equilibrium potential (Ex) for single ions The
equilibrium potential depends on: • Absolute temperature (T) • Valence of the diffusible ion (z) • Ratio of concentrations on the two sides of the membrane [x]inside / [x]outside Nernst egyenlet Nernst equation R * T ln ([Xo] /[Xi]) + z F (Eo - Ei) = 0 Eo - Ei = -(R * T / z F) ln ([Xo] / [Xi]) For practical use: Eo - Ei = (60 mV/ z) * log ([Xi] /[Xo]) Goldman Equation: Equilibrium Potential for Multiple Ions Generalization of the Nernst equation extended to include the relative permeability of each species of ion. Eions = -60 log PK[K+]i + PNa[Na+]i + PCl[Cl-]o PK[K+]o + PNa[Na+]o + PCl[Cl-]i PK, PNa, PCl = the permeability constants [K+]i, [Na+]i, [Cl-]i, [K+]o, [Na+]o, [Cl-]o = the intracellular and extracellular ion concentrations A nyugalmi membránpotenciál mérése Measurement of Resting Membrane Potencial A pozitív és negatív töltések megoszlása Distribution of positively and negatively charged ions Membránpotenciál Membrane Potential
• Arises when there is a difference in electrical charge between the two sides of the membrane. • Can be generated by active electrogenic pumping or by passive ion diffusion. • Membrane potential is determined by 3 main factors: 1. The concentration gradient of the monovalent cations 2. The selective permeability of the membrane for cations 3. The concentration of the intracellular, impermeable anions A nyugalmi membránpotenciál meghatározása Definition of Resting Membrane Potential An equilibrium condition, in which there is no net movement of ions across the plasma membrane, is known as the resting membrane potential. A nyugalmi membránpotenciál egyszerűsített modellje A Simple Model of the Resting Membrane Potential Cl- cc cc Na+ E E -70 mV EC IC Eeq (mV) Na+ 150 15 +60 K+ 5 150 -90 Cl- 125 10 -70 PK >> PNa cc E K+ Em = -70 mV A nyugalmi membránpotenciált fenntartó mechanizmusok Mechanisms Establishing Resting Membrane
Potential A passzív iondiffúzió szerepe a membránpotenciál kialakításában Role of Passive Ion Diffusion in Generating Membrane Potential • Membrane potential across the plasma membrane mainly (but not entirely!!) depends on the K+ gradient and the K+ channels. • To illustrate: – Suppose there is no voltage gradient across the plasma membrane (i.e the membrane potential is zero) – K+ is high inside and low outside. – K+ will leave the cell through K+ channels (known as leak channels) driven by its concentration gradient. Na+/K+ ATPase • Can cause a hyperpolarization (intracellular compartment becomes more negative) if its rate is increased. • Can be inhibited by cardiac glycosides (e.g digitalis) • Can be accelerated either by an elevation of the intracellular [Na+] or extracellular [K+]. • Is essential for maintenance of a resting membrane potential across a normal mammalian nerve or muscle cell membrane (directly, via its electrogenicity and
indirectly, via maintaining the Na+ and K+ concentration gradient) A nyugalmi membránpotenciál létrehozásáért felelős tényezők Establishment of resting membrane potentials in nerve fibers How would the leak of potassium from the cell be affected by Reducing the permeability of the membrane to potassium? Increasing the intracellular potassium concentration? Hyperpolarizing the membrane potential? Reducing the activity of the sodium-potassium pump? How would the leak of potassium from the cell be affected by Reducing the permeability of the membrane to potassium? Leak would be IMMEDIATELY reduced. Increasing the intracellular potassium concentration? Leak would be IMMEDIATELY increased. Hyperpolarizing the membrane potential? Leak would be IMMEDIATELY reduced. Reducing the activity of the sodium-potassium pump? Leak would NOT be affected immediately. (LATER, because of the loss of electrogenic force, a small depolarization would occur, which would increase the
leak of potassium. EVEN LATER, the membrane potential would dissipate, and this would reduce the potassium gradient, and reduce the leak of potassium.)
Concentration difference across the membrane ∆x: Thickness of the membrane Például For example If the extracellular concentration of a substance doubles from 10 mM to 20 mM while the intracellular concentration remains at 5 mM, the rate of diffusion increases by how much? Twofold? Threefold? Fourfold? Például For example If the extracellular concentration of a substance doubles from 10 mM to 20 mM while the intracellular concentration remains at 5 mM, the rate of diffusion increases by how much? Threefold: (20-5):(10-5) or 15:5=3 !!! Ozmózis a sejtmembránon keresztül Osmosis at the Cell Membrane van’t Hoff’s Law π = RT(ϕic) π : Osmotic pressure R: Ideal gas constant T: Absolute temperature ϕ: Osmotic coefficient i: Number of ions formed by dissociation of a solute molecule c: Molar concentration of solute Osmolarity: The sum of the molarities of all dissolved particles in a solution - ϕic! Isotonic ~300 mosmol/l Hypotonic < 300 mosmol/l
Hypertonic > 300 mosmol/l Osmosis Nyugalmi membránpotenciál Resting membrane potential Dr. Zsembery Ákos Example 1 • Membrane permeable to K+, NOT to Cl• Transmembrane potential is zero and the net flux is zero. • Why? For each K+ ion that moves in one direction, another K+ ion moves in the opposite direction producing equal concentrations of K+ in I and II Example 2 • KCl added to I, creating a positive potential in II • 2 forces on K+ ions – Chemical gradient favoring net flux of K+ from I to II – Electrical potential difference favoring net flux of K+ from II to I Example 3 • Build-up of pos. charge in II repels movement of further K+ ions from I • Negative charges in I hold K+ back with electrostatic attraction • Electrochemical equilibrium is reached and no net flux of K+ occurs • Equilibrium potential for K+ is established across the membrane Nernst egyenlet Nernst equation Equilibrium potential (Ex) for single ions The
equilibrium potential depends on: • Absolute temperature (T) • Valence of the diffusible ion (z) • Ratio of concentrations on the two sides of the membrane [x]inside / [x]outside Nernst egyenlet Nernst equation R * T ln ([Xo] /[Xi]) + z F (Eo - Ei) = 0 Eo - Ei = -(R * T / z F) ln ([Xo] / [Xi]) For practical use: Eo - Ei = (60 mV/ z) * log ([Xi] /[Xo]) Goldman Equation: Equilibrium Potential for Multiple Ions Generalization of the Nernst equation extended to include the relative permeability of each species of ion. Eions = -60 log PK[K+]i + PNa[Na+]i + PCl[Cl-]o PK[K+]o + PNa[Na+]o + PCl[Cl-]i PK, PNa, PCl = the permeability constants [K+]i, [Na+]i, [Cl-]i, [K+]o, [Na+]o, [Cl-]o = the intracellular and extracellular ion concentrations A nyugalmi membránpotenciál mérése Measurement of Resting Membrane Potencial A pozitív és negatív töltések megoszlása Distribution of positively and negatively charged ions Membránpotenciál Membrane Potential
• Arises when there is a difference in electrical charge between the two sides of the membrane. • Can be generated by active electrogenic pumping or by passive ion diffusion. • Membrane potential is determined by 3 main factors: 1. The concentration gradient of the monovalent cations 2. The selective permeability of the membrane for cations 3. The concentration of the intracellular, impermeable anions A nyugalmi membránpotenciál meghatározása Definition of Resting Membrane Potential An equilibrium condition, in which there is no net movement of ions across the plasma membrane, is known as the resting membrane potential. A nyugalmi membránpotenciál egyszerűsített modellje A Simple Model of the Resting Membrane Potential Cl- cc cc Na+ E E -70 mV EC IC Eeq (mV) Na+ 150 15 +60 K+ 5 150 -90 Cl- 125 10 -70 PK >> PNa cc E K+ Em = -70 mV A nyugalmi membránpotenciált fenntartó mechanizmusok Mechanisms Establishing Resting Membrane
Potential A passzív iondiffúzió szerepe a membránpotenciál kialakításában Role of Passive Ion Diffusion in Generating Membrane Potential • Membrane potential across the plasma membrane mainly (but not entirely!!) depends on the K+ gradient and the K+ channels. • To illustrate: – Suppose there is no voltage gradient across the plasma membrane (i.e the membrane potential is zero) – K+ is high inside and low outside. – K+ will leave the cell through K+ channels (known as leak channels) driven by its concentration gradient. Na+/K+ ATPase • Can cause a hyperpolarization (intracellular compartment becomes more negative) if its rate is increased. • Can be inhibited by cardiac glycosides (e.g digitalis) • Can be accelerated either by an elevation of the intracellular [Na+] or extracellular [K+]. • Is essential for maintenance of a resting membrane potential across a normal mammalian nerve or muscle cell membrane (directly, via its electrogenicity and
indirectly, via maintaining the Na+ and K+ concentration gradient) A nyugalmi membránpotenciál létrehozásáért felelős tényezők Establishment of resting membrane potentials in nerve fibers How would the leak of potassium from the cell be affected by Reducing the permeability of the membrane to potassium? Increasing the intracellular potassium concentration? Hyperpolarizing the membrane potential? Reducing the activity of the sodium-potassium pump? How would the leak of potassium from the cell be affected by Reducing the permeability of the membrane to potassium? Leak would be IMMEDIATELY reduced. Increasing the intracellular potassium concentration? Leak would be IMMEDIATELY increased. Hyperpolarizing the membrane potential? Leak would be IMMEDIATELY reduced. Reducing the activity of the sodium-potassium pump? Leak would NOT be affected immediately. (LATER, because of the loss of electrogenic force, a small depolarization would occur, which would increase the
leak of potassium. EVEN LATER, the membrane potential would dissipate, and this would reduce the potassium gradient, and reduce the leak of potassium.)




 Just like you draw up a plan when you’re going to war, building a house, or even going on vacation, you need to draw up a plan for your business. This tutorial will help you to clearly see where you are and make it possible to understand where you’re going.
Just like you draw up a plan when you’re going to war, building a house, or even going on vacation, you need to draw up a plan for your business. This tutorial will help you to clearly see where you are and make it possible to understand where you’re going.